Zhejiang University alumni enhance the efficiency of the Cas9 gene editing by a
During his Ph.D. and postdoctoral studies, he successively witnessed his mentors becoming Nobel laureates. Meanwhile, he published a Science paper and a Cell paper as the first author.
This is the unique experience of Chen Kai, an undergraduate alumnus of Zhejiang University and a Ph.D. graduate from the California Institute of Technology in the United States.
The aforementioned Cell paper was jointly completed by him, his postdoctoral mentor, Professor Jennifer A. Doudna from the University of California, Berkeley, and other colleagues.
In their research, they focused on the correlation between protein structure and function, and elucidated the key factors that regulate the efficiency of Cas9 gene editing at the molecular level, which will guide the development of new gene editing tools.
The improved CRISPR gene editor strategy proposed this time is expected to accelerate the development of gene editing tools to meet various application needs.In the future, they also hope that this strategy can be used to develop more universal and efficient gene editing systems.
Advertisement
Why is it difficult to improve the efficiency of Cas9 gene editing?
It is reported that the CRISPR gene editing system consists of two key components: guide RNA (sgRNA) and DNA endonuclease Cas protein.
The role of guide RNA (sgRNA) is to locate the target DNA sequence. Subsequently, the DNA endonuclease Cas protein (such as Cas9, Cas12) completes the cutting of the target DNA sequence.
This gene editing system has now been widely used in various directions of life science research and has great potential in treating human genetic diseases.Because of this, the academic community has been continuously exploring the naturally occurring CRISPR-Cas systems in the natural world over the past decade to develop gene-editing tools suitable for different ranges.
At the same time, many laboratories have also used protein engineering to modify the known CRISPR-Cas systems to improve their performance, such as expanding the recognition range of target sequences and reducing the risk of off-target effects.
However, there is still a lack of effective means to improve the gene-editing efficiency of Cas9.
On the one hand, it is because people are still unclear about the determinants of Cas9 gene-editing efficiency.
On the other hand, at the molecular level, people lack a clear understanding of the structure of the Cas9 protein, especially what kind of structural changes can improve the efficiency of Cas9.Proposing a Strategy to Modify the Cas Protein Wedge Domain
For a long time, Jennifer Doudna's laboratory has been dedicated to studying the biochemistry of microbial immune systems, as well as the development and application of CRISPR-based gene-editing tools.
In this project, they aim to study the performance of CRISPR gene editors and explore how to effectively modify gene editors to achieve better editing efficiency.
Therefore, in this study, they focus on the Cas9 protein (namely GeoCas9) from Geobacillus stearothermophilus.It has good thermal stability and could serve as a better gene editor. Unfortunately, the editing effect of GeoCas9 in mammalian cells is very poor.
Previously, Chen Kai had used the strategy of "directed evolution" to screen and modify GeoCas9, thereby obtaining a new mutant (i.e., iGeoCas9).
This not only greatly improved the efficiency of gene editing but also retained thermal stability.
For the directed evolution of proteins, it often obtains mutants with better activity through artificial random mutation and activity screening.
Throughout this entire directed evolution process, although the mutations of amino acids are random, Chen Kai paid special attention to the fact that the effective amino acid mutations that play the most critical role in improving efficiency are often concentrated in a previously little-noticed protein region - the wedge (WED, wedge) domain.Based on this, in this work, they conducted a detailed comparison of the differences in protein structure and biochemical activity between the two versions of GeoCas9 (wild type and modified version), focusing particularly on the changes in the WED domain and their corresponding effects on protein function.
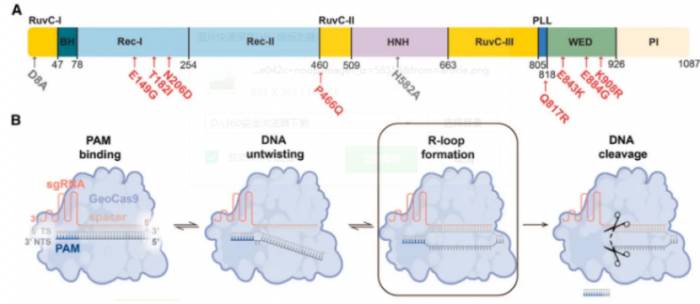
Taking iGeoCas9 as an example, three amino acid mutations in the WED domain will establish new interactions with the target double-stranded DNA, thereby enhancing the binding ability to DNA, and thus allowing iGeoCas9 to recognize a broader range of DNA sequences.
Then, they further compared the biochemical activities of the two GeoCas9s and carefully explored each elementary step of Cas9 function, as well as external influencing factors.
Through this, they found that: in every step of Cas9 function, such as from the initial recognition of the PAM sequence, to the initial bending of the target DNA sequence, and then to the unwinding of the double-stranded DNA, the effective amino acid mutations in the wedge-shaped domain can all play a role in enhancement.
Based on this, they proposed a strategy for modifying the wedge-shaped domain of Cas proteins to improve the efficiency of different gene editors and have been successful on different Cas9 proteins.For example, they introduced similar WED domain mutations into other Cas9 proteins, and the results showed that similar amino acid mutations could also increase the efficiency of gene editing by more than a hundred times.
The team also anticipated that this protein modification strategy could be more widely used to enhance the efficiency of other different gene editors.
Recently, the related paper was published in Cell with the title "Rapid DNA unwinding accelerates genome editing by engineered CRISPR-Cas9" [1].
Chen Kai is the co-first author, and Jennifer Doudna is the corresponding author.
During his Ph.D. and postdoctoral period, he has witnessed two mentors becoming Nobel laureates.Chen Kai stated, "This topic is very much in line with my personal scientific logic."
He established this logic on the following two points:
Firstly, to make new discoveries that change people's understanding of certain scientific issues (theory);
Secondly, to create new tools that change the way people study science (application).
He said, "In the previous topic, I developed new tools, modified new Cas9 proteins, and used them to develop new gene editors, while expanding downstream applications related to disease treatment."In this project, he has returned to a rational understanding perspective by exploring new laws and feeding them back into the development of tools.
"It also combines the protein engineering and directed evolution skills I learned during my doctorate. Now I apply them to my postdoctoral research, achieving an organic unity between theory and application," he said.
As mentioned earlier, Chen Kai graduated from Zhejiang University as an undergraduate and from the California Institute of Technology in the United States as a doctorate. During his senior year, he graduated with the highest score in the chemistry major.
During his doctorate, Chen Kai published a Science paper as the first author, which for the first time demonstrated that natural enzymes modified by directed evolution strategies can acquire unnatural functions for the synthesis of high-energy small molecular compounds.
In 2018, during his doctorate, Chen Kai's doctoral supervisor Frances H. Arnold won the Nobel Prize in Chemistry. In the Nobel Prize press conference report and Professor Arnold's Nobel Prize report, Chen Kai's work was cited.In 2020, Chen Kai joined the University of California, Berkeley to engage in postdoctoral research. "Here, I once again witnessed my mentor receiving the Nobel Prize in Chemistry, which is truly a fortunate event in life," he said.
Currently, he has led the completion of multiple projects during his postdoctoral period, including the development and modification of new gene editors, the development and innovation of gene editor delivery strategies, and research on the application of gene editing for the treatment of genetic diseases.
In the future, he also hopes to further develop gene editing tools and better apply them in the treatment of genetic diseases.